You see a bright shiny package at the garden center saying that it can help you have the most bountiful garden ever, the greenest lawn in the neighborhood, your plants will have miraculous growth, or it will supply every element on earth to make sure that your plants are living their best life. It’s got what plants crave….It’s got electrolytes! You reach out to grab that package and ……. Woah! Pump the brakes! Do you know if your plants even need to be fertilized? Are you just falling for that shiny marketing, or do your plants really need added fertility to grow?
It turns out that many gardeners add fertilizer out of habit or because a shiny package or advertisement told them they needed to do it. The fact is, though, that you may or may not need to add fertilizer to get your plants to grow healthy. It is actually more likely than not that the level of nutrients in soil is perfectly adequate for healthy plant growth. And guess what, there really is a way to know what plants crave…or at least are lacking: A soil test.
We here at the Garden Professors (and those of us who work in extension) often get questions or hear comments about gardeners adding fertilizer or random household chemicals and items to their plants and soils with no idea what they do or even supply. They’ll throw on the high powered 10-10-10, the water soluble fertilizer, rusty nails, or even (shudder) the oft mentioned Epsom salts because it is just what they’ve been told to do.
A few months ago, my GP colleague Jim Downer talked about why to amend soil– focusing mainly on organic material and a little bit of fertility. In this article, I’m going to share some how and what: what plants need in terms of nutrients, how to determine what nutrients you need to add, what you can use for increasing fertility (conventional and organic), and how to calculate how much fertilizer to add.
What plants really need
Plants have a number of essential plant nutrients that they need from the environment in order to properly grow and function. Hydrogen, carbon and oxygen are all important, but are not something that gardeners have to supply since they are taken in by the plant in the form of water and carbon dioxide (unless you forget to water your plants, like I sometimes do — but death will occur from dehydration well before lack of hydrogen).
There are six soil macronutrients, which means that they are used in larger amounts by the plants. These include nitrogen, phosphorous, and potassium, which form the basis of most common fertilizers that have those magic three numbers on them (example: 10-10-10). Those three numbers indicate that the fertilizer contains that percentage of the elemental nutrient in it. For this example, the fertilizer contains 10 percent nitrogen, 10 percent phosphorous, and 10 percent potassium. The other three soil macronutrients are magnesium, sulfur, and calcium. Depending on your location, your soil may be abundant or deficient in these nutrients, especially magnesium and calcium. Sulfur is commonly released during decomposition of organic matter, so it is usually present in sufficient amounts when soil is amended with (or naturally contains) organic matter.
If a soil is deficient in a nutrient that a plant requires it is usually a macronutrient since plants use them at higher levels. However, deficiency is still unlikely in most soils unless there is a high volume of growth and removal, such as in vegetable gardens and annual beds (or if you’re growing acres of field crops like they do here in Nebraska). These are also the nutrients that are most common on soil tests, since they are the ones that are used the most by plants.
Soil micronutrients are needed in much smaller amounts. Those nutrients are boron, copper, chlorine, manganese, molybdenum, and zinc (remember the periodic table?). These are also usually supplied from organic matter or from the parent soil material so deficiency is even less likely than for macronutrients. Tests for these aren’t usually part of a basic soil test, so if you suspect you might have a deficiency you might have to get a specialized test. There are some basic physiological signs of deficiency that plants might exhibit in response to specific deficiencies, but their similarity to other conditions make it an imprecise tool for diagnosing a deficiency.
Compost is a good source of nutrients, especially micronutrients (as we’ll read later). Using compost alone may be sufficient for many gardens, such as perennial beds. However, higher turnover and higher need areas like vegetable gardens may need supplemental fertilization beyond compost. That’s where the soil test comes in.
What’s on the menu….interpreting soil test results
If you’ve had your soil tested by a lab (which is recommended, since it is much more precise than those DIY test kits), you’ll get results back that give you the level of nutrients in your soil and usually recommendations for how much of each nutrient you need to add to the soil for basic plant health. This is a general recommendation that is common for most plants, which is generally sufficient for average growth. If the test says that the nutrient levels are normal, you don’t have to add anything….I repeat….YOU DON’T HAVE TO ADD ANYTHING. If it says you need one nutrient of the other you’ll need to add it to your garden or around the plant. As we’ve said before, disturbing the soil as little as possible is best, so if you’re using a fertilizer product aim for one that you can broadcast on top of the soil or is water soluble. This goes for compost as well – try to apply it to the top of the soil and it will incorporate over time.
 Your soil test results will usually tell you to add nutrients in pounds per a certain square footage. In the example pictured, there’s a recommendation of 3.44 lbs of Nitrogen per 1000 square feet. That number is for the actual nitrogen, and since different nutrient sources have different amounts of nitrogen you’re going to have to do some math to figure out how much fertilizer you need per 1000 square feet and then multiply that by how many thousands of square feet you have.
Your soil test results will usually tell you to add nutrients in pounds per a certain square footage. In the example pictured, there’s a recommendation of 3.44 lbs of Nitrogen per 1000 square feet. That number is for the actual nitrogen, and since different nutrient sources have different amounts of nitrogen you’re going to have to do some math to figure out how much fertilizer you need per 1000 square feet and then multiply that by how many thousands of square feet you have.
I’ll note here that soil labs do not usually test for nitrogen due to the variable nature of nitrogen in the soil and the lack of affordable or reliable tests. Nitrogen fluctuates widely over a short period of time and is not as persistent in the soil as other elements due to plant take-up, microbial action, and weather conditions. Nitrogen recommendations are usually made based on the crop indicated for the test and may be informed by the levels of other nutrients.
Let’s say that I’m using an organic fertilizer product I purchased at the garden center and the nutrient analysis is 4-3-3 (these numbers are standard for organic nutrient sources, which have lower nutrient levels than conventional fertilizers). That means that for every 100 lbs of that product, 4lbs are nitrogen, 3 are phosphorous, and 3 are potassium. My (hypothetical) garden is 10ft by 20ft, which is 200 square feet.
So we divide 200 by 1000 to get .2, which represents that my area is 20% of the area listed on the recommendation. If my garden were 3500 square feet, then that number would be 3.5.
Next, multiply the Nitrogen recommendation of 3.44 lbs by .2. This give me 0.688. This tells me that I need .688 lbs of nitrogen to amend my 200 square feet.
So I just need to weigh out .688 lbs of the fertilizer, right? Nope – we have to account for the fact that my fertilizer is only 4% nitrogen- only 4 lbs out the 100 lb bag. We can estimate amounts by figuring out how much nitrogen is in smaller amounts of the fertilizer. Since we know that 100lbs has 4lbs of N, then 50lbs has 2lbs of N, and 25lbs has 1lb of N. If I want to get a more precise amount of fertilizer poundage to get my .688 lbs of N, then we divide the pounds of N needed by the decimal percentage of N in the fertilizer. So that would be .688 / .04, which gives us 17.2 lbs of fertilizer.
Now, considering that the bagged product that I bought is $25 for 8lbs, I may want to reconsider using it for this application…unless I enjoy throwing my pearls before swine or I’m fertilizing my money tree.
If you do the math, you’ll note that this fertilizer will add more than the recommended amount of phosphorus and potassium. You’ll either need to decide if that is acceptable or if you need to find another source of nutrients.
If you’re not using a prepared fertilizer product but rather an organic source of nutrients, you can still calculate how much to add to get to the recommended amount. The following are some good lists of nutrient ranges of organic materials:
https://extension.psu.edu/using-organic-nutrient-sources
https://vegetableguide.usu.edu/production/soil-nutrient-water-management/organic-nutrient-sources
A note about pH
 Another thing your soil test will tell you is the pH of the soil. In general, plants prefer a soil pH just on the acidic side of neutral (between 6.0 and 7.0). There are plants that prefer different pH levels – such as blueberries and azaleas and their need for a more acidic soil between 4.5 and 5.2. Changes in pH affect the availability of nutrients to plant by affecting ionic bonds of the elements. For the most part, the nutrients are more available at that neutral pH. You’ll note that iron is more available at lower pH levels, which is why those acid-loving plants grow better at lower pHs – they’re heavier iron feeders.
Another thing your soil test will tell you is the pH of the soil. In general, plants prefer a soil pH just on the acidic side of neutral (between 6.0 and 7.0). There are plants that prefer different pH levels – such as blueberries and azaleas and their need for a more acidic soil between 4.5 and 5.2. Changes in pH affect the availability of nutrients to plant by affecting ionic bonds of the elements. For the most part, the nutrients are more available at that neutral pH. You’ll note that iron is more available at lower pH levels, which is why those acid-loving plants grow better at lower pHs – they’re heavier iron feeders.
If your pH is extreme in one way or the other, you’ll either need to find plants that thrive at that level or adjust the pH if that isn’t possible. To raise pH in acidic soils the most common method is application of lime. To lower pH, you’ll need something high in sulfur. For more information, visit https://articles.extension.org/pages/13064/soil-ph-modification .
Having a philosophical moment in the garden

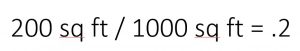

Good information but just a little off on the macronutrients. The three numbers represent the percentage of Nitrogen, Phosphate and Potash rather than Nitrogen, Phosphorus and Potassium. Elemental N is correct but not elemental P nor elemental K.
Jim- Thanks for your comment, however the information you provide is incorrect. The numbers do indeed represent elemental P and K. The myth/confusion comes from the usage of phosphate and potash as interchangeable with elemental P and K in the past. This is documented in both extension publications (https://extension.oregonstate.edu/news/what-do-numbers-fertilizer-packages-mean) and from fertilizer companies themselves (https://www.pennington.com/all-products/fertilizer/resources/understanding-fertilizer-labels).
No John you have it wrong. It’s no myth. Better check with your soils people if you don’t believe me.. Phosphorous is always on the label as phosphate ( P205 ) and potassium is always on the label as potash K20.
Jim, I suggest you read the links that John provided, rather than argue the point without evidence.
Jim,
After logging back in and seeing your comments I did a little digging (ha). It does seem that there is confusion on the language and use of elemental P and K vs Phosphate and Potash on the labels. You are correct that the label does indeed state Phosphate or Potash. As the text that Linda cites downthread points out, it is a bit outdated and seen as confusing. Especially since it is often referred to as “phosphorous expressed as P205” and soil test recommendations can either be expressed as a need for elemental P or for P205. It gets even more confusing when you consider that the analysis for many organic amendments are expressed as elemental P and K – which is where my confusion was coming from. I think we can all agree that it would be better if we could figure out the semantics and apply them more evenly. I’ll add a note to the article. Thanks for sharing.
Without evidence? I’ve been in commercial horticulture for 30 years. This is not ‘argue the point without evidence’. It’s basic soil science! In the greenhouse business I need to calculate elemental P and K often. if you need some help with NPK go to Dr. John Erwin’s tutorial from the University of Minnesota (department of horticulture).
In this group, published, peer-reviewed evidence is the standard. Anecdotal information counts for little. Give us a link to a recent, relevant publication to support your statements.
In the meantime, look at this publication from WSU, written by soil scientists, and peer reviewed. Look at page 11, which reads
“Historically, the amount of phosphorus in fertilizer has been expressed not as P, but as units of phosphate (P2O5). Similarly, fertilizer potassium is expressed as potash (K2O). This practice is still used for fertilizer labels and recommendations, even though there is no practical reason for the system, except that people are accustomed to it. (If there is a need to convert from P to P2O5, the conversion is 1 lb P = 2.3 lb P2O5. For potassium, the conversion is 1 lb K = 1.2 lb K2O).Thus, a bag of fertilizer labeled 12-4-8 contains 12 percent nitrogen, 4 percent phosphorus expressed as P2O5, and 8 percent potassium expressed as K2O. This information on chemical content is called a fertilizer analysis. The analysis for processed fertilizers guarantees the amount of available nutrients in the fertilizer, whereas the analysis for organic fertilizers represents the total amount of nutrients, rather than the amount of available nutrients. Because nutrients in most organic fertilizers are released slowly, the amount of immediately available nutrients is less than the total amount.”
http://cru.cahe.wsu.edu/CEPublications/EM063E/EM063E.pdf
Thank-you. Your page 11 from WSU states exactly what I have been saying.
I think you’re seeing what you want to see Jim. Historically means “in the past.” They say there is no practical reason for it, but the primary elements are as John described them in this article..
Poor Jim
He makes a rather pedantic remark about a technical matter and the roof falls in.
Of course he is right and the fertiliser bag gives analyses expressed in the outmoded convention of expressing phosphate and potassium as it it was an oxide.
It is of no significance to gardeners as recommendations for fertiliser use also use the same convention.
I find your reply to Jim a little pompous and it always grates with me when you repeat the mantra of evidence based research.. Science base yes and of course evidence too but not that based on a single piece of research as is often the case.
If you look at most major fields of research such as medicine or nutrition there are hundreds of projects of peer based research that is often entirely contradictory. So too in horticulture.
No to believing gardener’s tales but do listen to expert opinion (and question that too).
Hi– just a hobbyist here (my psychology degrees don’t help in the greenhouse). I’m curious how these principles would apply to hydroponic growing conditions. I grow a lot of ornamental plants in substances like sphagnum moss and perlite. Would those always require fertilizer? I’ve also wondered a great deal about epiphytic plants–have they evolved to not require as many nutrients, or are the rather romantic stories about wild epiphytes catching bird poo true? 😉
You’re right that growing plants in non-soil media like sphagnum or perlite would require the use of fertilizer. You can think of it as a hydroponic or semi-hydroponic method. Epiphytes do collect some nutrients from dust and dirt particles, from the rain water (which would pick up nutrients from dust and dirt), and maybe even from a well-aimed bird dropping. They’ve evolved to be adept at absorbing nutrients from things other than soil (and when you think about it, it is the water that plants uptake that moves the nutrients into them – they don’t just absorb them from the soil).