Earlier this spring, I posted an article about seasonal climate forecasting and noted that we expected to see the development of an El Niño after three years of La Niña conditions ended in March 2023. And sure enough, an El Niño was declared in August 2023 and has been strengthening ever since. It has a 71% chance of becoming a strong event by December or January before starting to weaken, as they usually do in spring or early summer. In today’s post, I will remind you all what El Niño is and how it is expected to affect our climate this (Northern Hemisphere) winter and spring. That will affect how our gardens survive the colder conditions and prepare for next year’s growing season.

Refresher: What is El Niño?
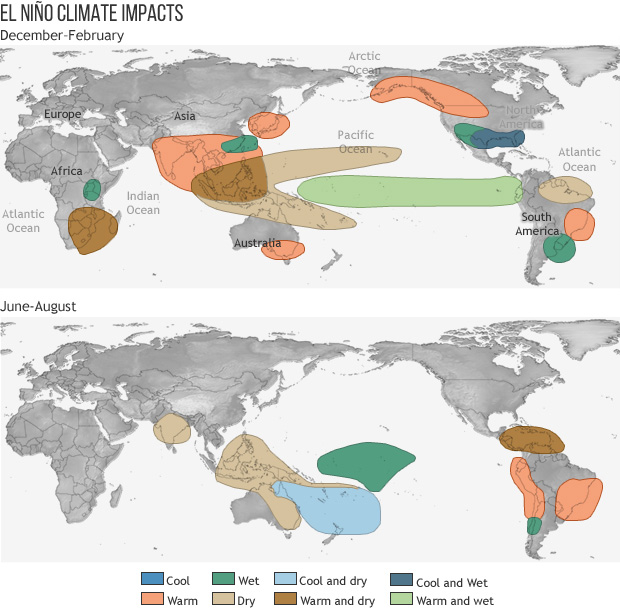
If you are a new reader of this blog, you may be wondering what El Niño is. El Niño and its companion, La Niña, are two opposite phases of an oscillation in atmospheric and oceanic weather patterns linked to the water temperature in the Eastern Pacific Ocean (EPO). When the ocean there is warmer than usual as it is now, rising air over the warm water creates thunderstorms which can affect the movement of global air currents that bring stormy weather to parts of the earth while leaving other areas high and dry. When the ocean there is cooler than normal in the La Niña phase of the pattern those currents shift, changing the expected weather pattern to something quite different resulting in a different pattern of temperature and precipitation than in El Niño . The maps below show how the climate changes globally in an El Niño in the December-February and June-August periods, corresponding to Northern and Southern Hemisphere winters, respectively. The El Niño pattern is linked to a variety of unusual weather phenomena around the globe. The variations in temperature and rainfall are what affect how our gardens grow or rest in preparation for the next growing season.
What is the current state of El Niño and how is it changing?
Right now, ocean temperatures in the EPO are from 1 to 3 degrees C ( 2-5 degrees F) warmer than normal all the way from the west coast of South America all the way west to the International Date Line. This is a large area of very warm conditions that are being heated even more by the trend towards warmer temperatures due to increases in greenhouse gases in the atmosphere. The heated water provides a lot of water vapor to the atmosphere that helps fuel thunderstorms and tropical systems. Normally in an El Niño year the number of tropical cyclones in the Eastern Pacific is larger than the number in the Atlantic because of that pool of warm water. This year the Atlantic has near record-setting sea surface temperatures which are helping to produce one named tropical storm after another (today we are have “Philippe” and “Rina” active in the Atlantic but only through the name “Kenneth” in the Eastern Pacific). Global warming has affected our climate patterns to such an extent that what used to be established El Niño and La Niña patterns are less likely than in previous decades, although there have always been variations from one event to the next.

What will happen from N. H. winter through spring?
According to the predictions of how the current El Niño will evolve, we can expect the current pool of warm water and the associated global weather patterns to last through at least the April-June period. Since El Niño seldom lasts for longer than a year we are likely to go back into neutral conditions after that and neither El Niño nor La Niña will dominate. This means that over the next few months we can expect the southern part of the United States to be cooler and wetter than normal since in El Niño years the jet stream is positioned over that part of North America. As storms are pushed through the region rainy and cloudy conditions keep daytime temperatures cool as precipitation in the form of rain or sometimes snow or ice falls. In northern parts of the United States extending north into Canada, warm and dry conditions are likely to lead to a lack of snow cover and shorter ice coverage on what are usually frozen lakes. Warmer than normal conditions are also likely to occur in most of Southeast Asia stretching from India to Japan. Drier than normal conditions are likely in the Western Pacific Ocean and in southern Africa as well as South America, leading to the possibility of droughts in those areas.

What does this all mean for our gardens the next few months?
In the parts of the world that are under the jet stream, cooler and wetter than normal conditions should lead to high levels of humidity and increases in soil moisture over the winter since evaporation will be low in the cold winter months. That means gardens in those areas should be fairly wet going into spring. That means a spring or summer drought there will be less likely than after a La Niña winter, but it could be muddy working in your garden areas next planting season. Since El Niño is already strong and getting stronger this wet winter pattern may start early this year so don’t dawdle in preparing your fall garden for winter since it might be hard to work in those wet conditions. Soil temperatures may stay cool later in the spring, delaying planting of seeds and vegetables or flowers that require warm ground to germinate and grow.
If you are in northern parts of the United States and up into Canada, you can expect warmer and drier conditions than usual. That could mean a lack of snow cover and loss of some plants that need insulation provided by the snow to survive the winter. Even though temperatures will be overall warmer than in non-El Niño years, there are still going to be cold outbreaks that can cause damage to plants that are over-wintering. The lack of precipitation could also lead to dry soil conditions in spring that could require increased irrigation or hinder the growth of seeds or new seedlings you might plant. The lack of soil moisture could also contribute to the development of drought later in the growing season.

Of course, even though El Niño and La Niña are the most reliable predictors for climate several months ahead, there are always other factors that can affect climate patterns too. There is no guarantee we will see these exact patterns this winter and there are sure to be some surprises that we don’t expect.