When I first started reading extension recommendations for plants around 15 years ago now, I read a lot about “balanced fertilizers”. Today I still see balanced fertilizers recommended, but not nearly as frequently, which is a good thing.
At this point you may be asking yourself “what is a balanced fertilizer?” A balanced fertilizer is one that has three numbers on its label which are the same — such as a 10-10-10 or a 14-14-14 (Nitrogen, Phosphorus and Potassium are the three nutrients indicated by
these numbers). Although it might seem like equal numbers would mean equal amounts of these nutrients, these numbers actually indicate percent Nitrogen, percent P expressed as P2O5 (in other words, if you took all of the phosphorus present in the fertilizer, made it into P2O5 and then added it back to the fertilizer and figured out what percent of the fertilizer that made up — that would be the second number) and percent potassium expressed as K2O (basically the same as the phosphorus example you saw previously).
But here’s the problem. The amount of phosphorus, and often potassium, that is added when you use a balanced fertilizer is typically out of line with the amount that the plant needs. This is because fertilizers are usually applied based on the amount of nitrogen that a plant needs. The reason that a balanced fertilizer was usually recommended was that phosphorus and potassium levels in these fertilizers is high enough that they provide all of those elements that a plant needed without being toxic to the plant. The problem is that, while the levels of these nutrients added might not be toxic, they are in excess of what is usually needed.
OK, so we’re adding excess phosphorus and potassium, what’s the problem? Well, for the potassium the problem isn’t usually that big a deal. In fact, a fertilizer bag with the first and third numbers equal may be what’s called for in many cases — fruits and nuts in particular often like a higher level of potassium. Additionally, the world has a pretty big store of potassium so we’re not likely to run out any time soon. Phosphorus, on the other hand, is a little bit different.
Phosphorus is a bit more hard to find in large quantity than either of the other elements in a bag of fertilizer. Because of this it is often the element that limits the growth of plants, for example algae. When phosphorus runs off into a lake or other body of water it can allow algae to go crazy and use up all of the oxygen in the water killing fish and other creatures (actually it’s the dead algae that do this — bacteria use oxygen while breaking the algae down). Most of you are familiar with this and know that it’s the reason why Minnesota and now Wisconsin have banned the use of phosphorus fertilizers on lawns without a soil test.
But here’s what you may not know. Most of the phosphorus which we use for fertilizers comes from rock phosphate. Rock phosphate is mined in only a few places around the world, Florida being one. Just as it is a foregone conclusion that we will run out of oil someday, it is also a foregone, but lesser known, conclusion that we will run out of rock phosphate. Estimates are that we will reach “Peak Phosphorus” (in other words maximum phosphorus production.) in 20 years or so and that we will run out in 50 – 100 years. Here’s an interesting article on the situation http://www.foreignpolicy.com/articles/2010/04/20/peak_phosphorus
So conserve our natural resources and skip the balanced fertilizer. When asked for a general use fertilizer recommendation I usually recommend something with a ratio of roughly 5-1-2, with a higher potassium content if you’re growing fruits or nuts.
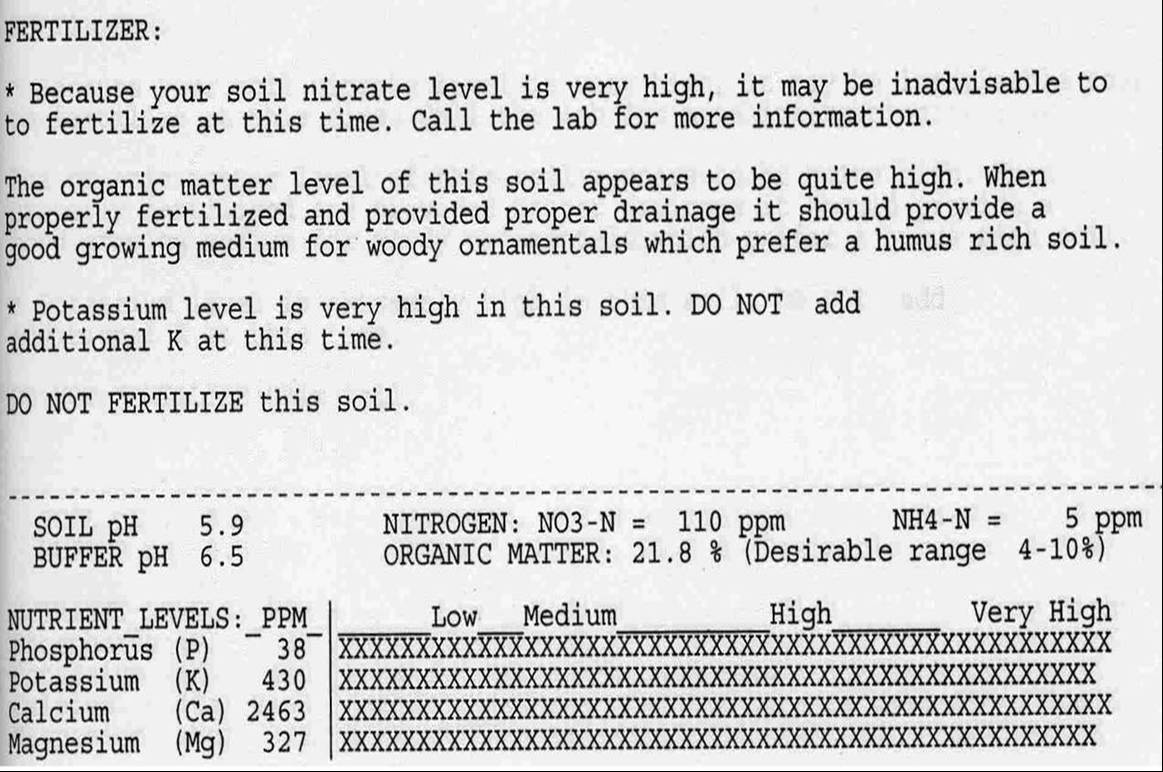 Figure 1. Note the high level of organic material in this soil, which contributes to the nutrient overload.
Figure 1. Note the high level of organic material in this soil, which contributes to the nutrient overload.