Minnesota, and I were cruising through old pictures and files and getting all sentimental about the cool stuff we used to do. A lot of it was never published just because after we were done with one thing we were just too damn excited to move on to the next. Anyway, one of the neatest experiments that we never wrote up was a phosphorus experiment. Here’s what it looked like to the casual observer.

Now let me explain the neat part to you a little. Inside those boxes, underneath three of the six plants in each container, are vials set up like this – three vials per plant (the black tubes provide air to the vials).

Each plant had one root placed into each of the three vials – one vial contained 1 ppm phosphorus, one vial contained 10 ppm phosphorus, and one vial contained 30 ppm phosphorus. The tub itself was also filled with one of these three solutions (1, 10, or 30 ppm phosphorus) as seen below.

At the end of the experiment we weighed the roots filling each vial, as well as weighing all of the roots from each plant. Here’s what we found for the individual vials.
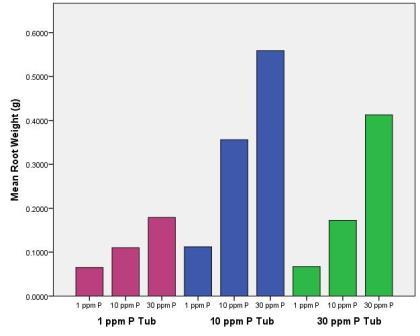
As you can see, more phosphorus in a vial meant that the plant would devote more energy to growing roots there – but also notice that the 10 ppm solution has the greatest mass of roots overall. Here’s what we saw when we looked at the total size of all of the roots from plants for the different solutions.
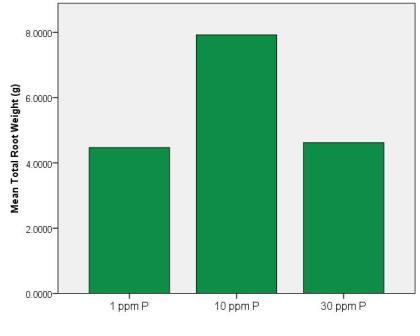
As you can see, the roots from the plants in the 10 ppm solution are the largest (shoots showed the same trend). So here’s the way I see it (this is the Big Mac part). I love Big Macs. If I see a McDonald’s I want to go in there – I gravitate towards McDonald’s to get Big Macs. But too many Big Macs aren’t good for me. They might even stunt my growth! It’s the same for phosphorus. Roots do grow towards phosphorus (this isn’t technically correct, but it works for my analogy so I’m sticking with it!), but that doesn’t mean that a tremendous amount of phosphorus is actually good for them. In fact, it might even stunt their growth! This could be for a variety of reasons, but most likely because the phosphorus would interfere with the uptake of other elements.
Good and interesting info. I understand natural forms of phosphorus are not in great supply in the environment, so that is probably a good thing. I would be curious to know what the results were for root crops.
On the contrary – there’s lots of phosphorus available in soils, especially nonagricultural soils. That’s why it’s so important to have a soil test done before you add any more phosphorous to your garden or landscape. In Jeff’s analogy, this would be like giving someone a Big Mac when they had already eaten 10 of them – unbeknownst to you.
One thing that we’ve discussed before in this blog is that excess phosphorous inhibits mycorrhizal fungi. More than anything else, a robust network of pla
nt roots and mycorrhizal fungi are crucial for plant health.
Why would these roots have different weight for the same plant?
Are you saying that phosphates are not only nutrients but also messengers?
In principle I’ve heard of ATP signaling…
Alternatively there is an awful lot of non-equilibrium in nutrient distribution from roots to other roots…