Upon reading this post’s title, you may be inclined to stop right there. (That’s why I have an eye-catching photo to lure you in.) While logic may seem irrelevant to your enjoyment of gardening, I can guarantee that reading this blog post will challenge many seemingly logical assumptions you’ve heard or read about. Recognizing unsubstantiated assumptions and avoiding their pitfalls means you can make wise choices about how you care for your gardens and landscapes.
A few definitions are needed before we get started:
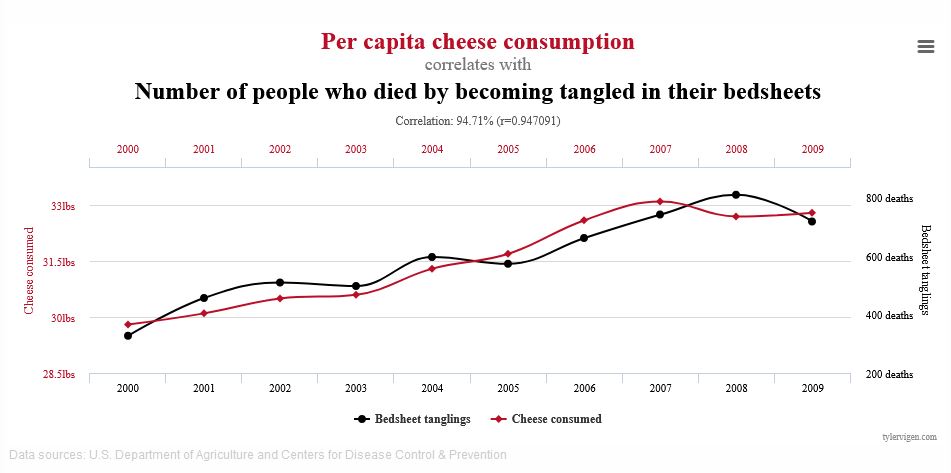
Correlation refers to variables whose changes mirror one another. For instance, the addition of nitrogen fertilizer to container plants is correlated to plant growth: as nitrogen levels increase so does plant growth. You can also have inverse correlation, where the variables move in opposite directions. An example is water availability in soil and planting density: the more plants you have in a specified area, the less water is in the soil.
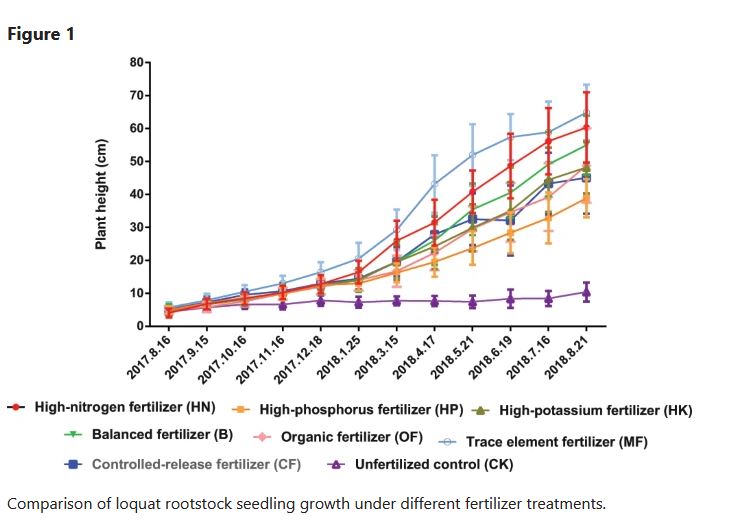
Causation takes correlation one step further: it establishes that one of those variables is causing the change in the other. Using the same examples, we know through published evidence that the increase in nitrogen is causing the increase in plant growth, and the increase in planting density is causing the decrease in soil water because of competing roots. These relationships are obvious to us, but what’s important is that these causative effects have been established through scientific experiments.
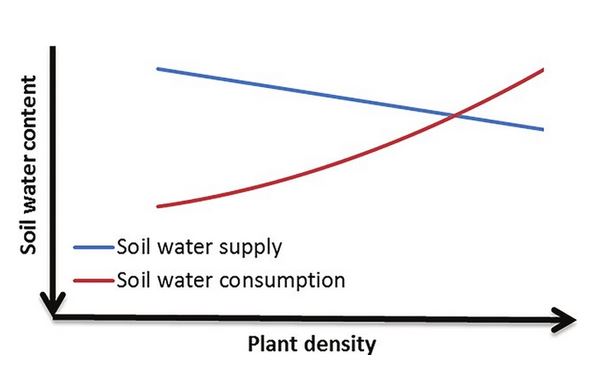
Sometimes scientific evidence doesn’t exist to demonstrate causation. That may be because it’s impractical or impossible to run an experiment that tests for a causative effect, or it may be because the experiments just haven’t been conducted yet. The latter is the unfortunate reality for those of us interested in managing gardens and landscapes: there is no major funding agency that supports field research for us. There is research being done, but it’s on a small scale with a shoestring budget…so the body of literature develops very slowly. In such situations, we must rely on established applied plant physiology and soil science to ask whether a suggested correlation might be elevated to causation.

Which brings me to my current source of online irritation: the constant blaming of tree failure on mulch volcanoes. Yes, tree failure is definitely correlated with mulch volcanoes – because lots and lots of newly planted trees fail. But is the mulch to blame? No one seems to care much that there is NO published work to show that mounds of appropriate mulch materials will somehow kill otherwise healthy trees. Instead, observers jump to the conclusion that thick layers of wood chip mulch kill trees. They are elevating correlation to causation in the absence of either experimental research OR known plant physiology. In fact, there is published research to show that thick layers of arborist wood chip mulch enhance tree establishment and survival. And there are many poor planting practices that increase the likelihood of tree failure. But it’s easiest to blame the wood chip mulch, though it’s merely masking a multitude of planting sins.

Street volcano 1 
Street volcano 2 
Volcano as garden fashion statement
Not interested in mulch volcanoes? Well, there are lots of other examples of garden and landscape management practices or phenomena that fall into the logical fallacy camp. I’ve linked to appropriate references, when available, that go into more detail:
- Black walnut allelopathy;
- Companion planting;
- Hot-weather watering and leaf scorch;
- Hugelkultur;
- Humus formation and importance in soils;
- Lasagna mulching;
- Native plant superiority;
- and just about any gardening product you can think of where there is NO published evidence – or appropriate, established plant or soil science – that supports any causative, beneficial effect on plants or soils. Cornmeal, Epsom salt, gypsum, and kelp products are just some of these.
All of these products, practices or phenomena are correlated with some anecdotal observation (increased yield, healthier soil, plant failure, etc.) that elevates them to causative relationships. But no science.

I’d encourage you to think objectively about your closely held beliefs about your gardens or landscapes. Are you sure that what you’re doing is actually beneficial? How do you know there’s a cause-and-effect relationship? I’m not going to talk you out of your cherished beliefs – but if you are a science-based gardener, you might talk yourself out of them instead.

What is its source of anguish?
More research needed!