Last month we discussed the various heavy metals that might end up in your garden and landscape soils. Today we’ll consider how different factors can alter heavy metal uptake by plants.

First of all, let’s consider plant uptake. Plant roots can either accumulate a particular metal or exclude it. If they exclude it, that’s the end of the story, (though it’s still a soil contaminant). If plants take it up, they can either store it in their roots, or they can transport it to some other part of the plant – stems, leaves, flowers, and fruits are possible destinations for metals in accumulator plants. Accumulation varies with plant species and life stage; in other words, seedlings may have different uptake abilities than later life stages. And of course, whether a plant accumulates or excludes a particular heavy metal does not mean the same uptake pattern holds for other heavy metals.

Secondly, soil conditions will influence heavy metal mobility. Heavy metals are positively charged, so anything in the soil that carries a negative charge – like clay particles and organic matter – will tend to hold heavy metals in place. That can either be good or bad, depending on your use of the landscape. If you are growing edibles, metals that are tightly bound to the soil are less likely to be taken up. But this also means that they are pretty much there to stay. Sandy soils don’t hold metals well, since sand particles carry no charge, so heavy metals are free to move elsewhere – into the air, into bodies of water, or into plant roots.
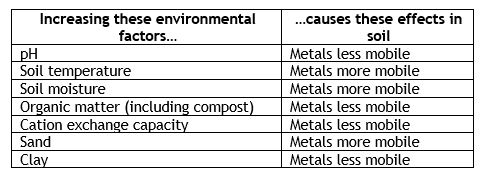
Additions of fertilizers, like those that contain phosphate or that chelate metals, will also increase the ability of plants to take up heavy metals. Likewise, earthworms ingest metals and bind them to other compounds that can be taken up by plant roots. And microbes associated with the roots (and the roots themselves) can acidify the rhizosphere, solubilizing metals and making them easy to incorporate.

It’s apparent that many factors are at play in determining whether plants will take up heavy metals, thus making it impossible to come up with lists of “safe” plants. There are hundreds, if not thousands, of studies on heavy metal uptake of vegetable and other crop plants worldwide, and the variability among their results is a direct reflection of the complexity of soil environments and plant physiology. Nevertheless, there are some very general observations about accumulator species that can be gleaned from the research:
- Roots are the most likely tissue to contain heavy metals, since they are the point of uptake; arsenic can accumulate in carrots and lead has been found in carrots and potatoes;
- Stems are much less likely to accumulate heavy metals, as they are basically just a straw connecting roots to leaves and other terminal tissues;
- Leaves, including basil, lettuce, and spinach, can accumulate heavy metals. Moreover, it appears that red leafed cultivars may accumulate more than those that are green leafed;
- Flowers and fruits, including vegetable tissues that produce seeds, are less likely to accumulate heavy metals. For plants that depend on animals to spread their seeds by ingesting the surrounding fruits and then excreting their seeds, it would be an evolutionary disadvantage to have those tissues carrying toxic heavy metals. That being said, there are vegetables, like beans, broccoli, and zucchini, that can accumulate heavy metals such as lead and arsenic.

By this point, I think we can agree there will never be a “one size fits all” approach to gardening safely when heavy metals are part of the soil, water, or air environment. Next month I’ll provide suggestions on how to navigate the confusion and design your own approach to creating gardens and landscapes that work around heavy metal contamination.




One thought on “The complicated issue of heavy metals in residential soils, part 2: How plant species and environmental variables complicate the issue”