Posted by Bert Cregg
We had a question on the Facebook site regarding fall fertilization of landscape plants. Fertilization in general, and fall fertilization in particular, is a complex topic and needs a little more room for explanation than the Facebook discussion allows.

As a general rule, most landscape trees and shrubs can maintain acceptable growth and appearance without fertilization. There are a couple of reasons for this. As Linda noted in the Facebook discussion, woody plants are fairly efficient at internal nutrient recycling. I’ve done a couple of studies where we sampled leaves of hardwood trees during the season and then re-sampled right after senescence and about 50% of leaf nitrogen is re-absorbed by trees before they fall. Conifers are even more efficient at conserving nutrients than hardwoods since they typically only lose 1/4th of their needles (or less) each year. In addition, many landscape trees are able to utilize fertilizer that is applied to surrounding turf. On the flip-side, nutrients that occur in litterfall are removed from the nutrient cycle in many suburban landscapes and this may eventually contribute to deficiencies.

Bottomline, landscape fertilization should be based on need; which can be assessed based on soil sampling, foliar sampling, or visible symptoms. At least two of the three methods should be employed to make a diagnosis. Each method has drawbacks and visible symptoms are usually the least useful since many nutrient deficiencies have similar symptoms or the symptoms may not be nutrient-related at all. In our area the only nutrient problems I am comfortable diagnosing based on visible symptoms are iron chlorosis in pin oaks and manganese deficiencies in red maples, both of which are induced by alkaline soils, not a lack of those particular elements.
So assuming we’ve established that fertilization is needed, what about fall fertilization? There are a couple of arguments that are usually brought forth for fall fertilization. One is that trees can absorb nutrients during the fall and then use them for spring growth. This is generally true provided that soils are warm enough to allow continued root growth and absorption. Another argument is that fall-applied fertilizer that is not taken up by roots in the fall be will available for uptake when soils warm again in the spring. A third, and less scientific reason, is that fall is often a slow time for arborists and landscape companies and fall fertilization is an easy service to add to their sales program.
There are a couple of objections that are usually raised to fall fertilization. One is that nutrients will leach through the soil over winter before they can be absorbed. This is one of those ‘it depends’ scenarios. If a nitrate-based fertilizer source is used, this is possible since negatively-charged nitrate anions won’t bind to negatively-charged cation exchange sites in the soil. If the nutrient source is urea or ammonium-based, the amount lost will be dependent on temperature since this will drive the conversion from ammonium, which can bind to cation exchange sites, to leachable nitrate.
The other usual objection to fertilizing trees in the fall is that it will reduce cold hardiness. There is no clear evidence to support this, however. Harold Pellett and John Carter at the University of Minnesota compiled dozens of studies on the effects fertilizer on plant cold hardiness (Horticultural Reviews 3:144-171). For conifers and temperature hardwoods they found no clear trend across studies, except that fertilizing with potassium improved cold hardiness is most cases (see table). The common perception that fall fertilization, especially with N, will increase cold damage probably stems from studies of fertilization of turf, which had negative impacts in 26 out of 29 studies cited by Pellett and Carter.
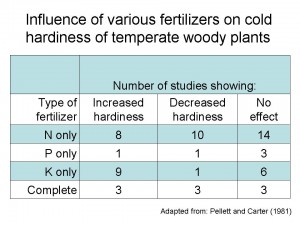
In summary, landscape trees and shrubs should be fertilized only where there is a demonstrated need. Fall is a good time to fertilize provided you avoid nitrate-N sources that will be prone to leaching.
I’m interested in your comment about the studies regarding fertilization of turf. We’re in central Virginia (Zone 7) where the recommendation is for fall fertilization. Our winter temperatures rarely go below 10 degrees F. If you would direct me to these studies I would appreciate it. I would especially like to know where they were conducted. This is the first I’ve heard about N increasing cold damage in turf grass.
Does Urea get converted to nitrate in the fall? At what temperature does this conversion stop or slow significantly?
Like many biological functions, nitrification is highly temperature dependent and increases exponentially between ~4 deg. and 25 deg. C.
Please note that is squiggle (~) to indicate approx. 4 deg. not a minus sign.
Why fertilize at all? Maybe the first year to help it get established. It must be forty some years since I fertilized trees or shrubs
How late is too late, assuming that even a low nitrogen fertilizer will stimulate some growth? Thanks in advance!
Paige:
The timing will depend on where you’re at in the country. If you have good bud-set, short day-lengths and cool temps you shouldn’t have any issues with re-growth. Around here (Michigan) I would say later Sept./early Oct. Much later and the plants won’t have much chance to take it up.