I think the blog and garden professors web page is pretty full of research and benefit descriptions of mulching, particularly with arborist chips. A little less clear is the role of amendments in garden soils. I always like to ask the “why” questions for gardening practices. Like “why” prune trees? Why fertilize, etc? Ideally gardening practices should be founded on a basis of science and inquiry as to their necessity. Poor structure early structural training or a damaged canopy may prompt tree pruning, mineral nutrient deficiency symptoms may suggest

fertilization. So why amend your garden soil? For me as a gardener you do this because your soil is not providing something you think your garden or plants growing in that soil need. This could be nutrients, or water. Amending as a soil or garden practice is best done in garden beds that host annual plants: vegetable gardens, color beds with annual plants etc. Obviously we are not going to lift all the perennials just to add some amendment under their root systems. We can also make arguments that amending soils that you are going to plant perennials in is unlikely to be helpful. So why amend?
Research on providing amendments in the holes of perennial plants has most often shown no significant differences compared to plants installed with just native backfill. There are lots of reasons for this and I can imagine some soils where amendments would give a slight boost, but these were not the soils most studies used (extreme sands). Mostly, perennial roots do not reside in the planting hole for long, so the time that amendments would be effective is very short. Since amending can also harm some plants if done incorrectly, University of California does not recommend the practice, neither did Harris for trees, shrubs and vines. Nor did we find any effect in palms (Hodel and others). For this blog we will assume that amending is restricted to annual plant beds. So why amend? The usual reason I hear is: “My soil is crap!” I think it would be interesting to survey people about their soil and see what they think about it as compared to how it actually grows. Most people seem to disparage their poor soil….So many gardeners believe that if they add something to their crap soil it will get better. Maybe, or no. Amending has potential benefits and detriments depending on what and how much you amend with.


So the potential benefits of amending are:
• rapid (immediate) modification of the planting bed
• potential increase of moisture holding capacity in the soil
• potential increase of nutrients and nutrient holding capacity (cation exchange capacity of the soil
• change in the soil texture, porosity
• rapid increase in soil organic matter
• suppression of soil borne pathogens
Potential detriments include:
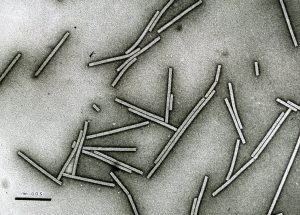
• nutrient draw from the soil (nitrogen immobilization: see images above)
• toxicity from residual phytochemicals
• adding pests (weeds or root pathogens)
• soil structure is destroyed
• soil food web is challenged and harmed
• may increase soil salinity
• contaminants can be transferred to gardens
One of the incontrovertible facts about amending is that you have to disturb the soil to do it. Amending involves digging in, roto-tilling, soil turning with a spade, or some kind of incorporation process. Usually the more the better. This destroys soil structure and a good part of the soil food web. Beneficial nematodes are highly sensitive to tillage and many are killed by tillage, often perturbing the entire soil food web in a disturbed soil (See research by Howard Ferris). The beauty of mulching is that the soil structure is not initially influenced only assisted in its further development. The downside is that mulches take months or years to have their effect. While amending can give immediate physical, chemical and biological assistance to soil, it also may bring pathogens, salts, toxic phytochemicals or weeds to your garden depending on what you choose to use as an amendment. Soil tends to be very resilient, so structure destroyed by amending is usually back in place at the end of the cropping cycle in many cases. The need for further amending should be considered carefully after each rotation.
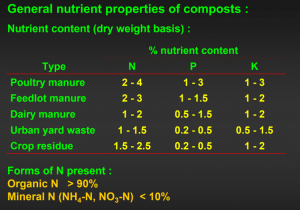
So you still want to amend? What do you amend with? In the case of mulching we (Garden Professors) make a strong argument for freshly prepared (not composted) arborist chips. Nothing could be worse for amending (at least in the short term). Un-decomposed substrates such as wood chips are high in carbon and low in nitrogen. They will cause the microbial community of soil to attack the carbon and utilize all the free nitrogen in the soil, screened or fine materials after composting have greater surface area and will enhance the water absorbing and nutrient holding properties they give to soil. Furthermore, well composted substrates or feedstocks will be free of pathogens and other pests so they should be “safe” for your garden. Composts that are made from plant feedstocks tend have concentrations of plant required minerals. Since composts lose about 2/3 of their carbon and moisture during the decomposition process they have a much higher mineral content than their feedstock. Manures have already been partially composted by the animals that made them. Additional composting increases their salt levels sometimes to plant damaging levels. Manures should be used with care, or in lower quantities as they can damage sensitive plants. Some manures and composts can be contaminated, since some long term soil-residual herbicides such as clopyralid are not broken down in the composting or animal eating process.


Gardeners are inventive in their use of compost and amendments, so there are many ways to amend soil. Peat moss has been the gold standard amendment for many gardens. However due to environmental damage, sustainability of peat moss use is questionable. Coco fiber or coir is a good amendment, but it can bring high salts with it depending on how it was processed. Biosolids are phenomenal amendments and often produce growth responses, but there can be issues with metals and other biological contaminants in biosolids. Home-made compost is a good amendment because you know what is in your compost, as long as it is properly prepared and cured, it can function very well. Greenwaste or yardwaste compost is a possibility, but from my experience, most of these if commercially produced are not well prepared, and are not mature (they still heat up if piled). Many gardeners like coffee grounds, and with the advent of large coffee companies, grounds may be available in bulk quantities. Be careful though as some sources of coffee are toxic to many plants and their use should be limited in any amending situation.
What about rate? In my research I have always tried to amend soils 50% by volume. So a three inch layer of amendment tilled six inches deep has been my goal. Most annual plants have their roots in the upper six inches and this strategy works well. Also, most rototillers are only good for about a six inch depth. If you intend to dig deep with a spade and incorporate to depths beyond six inches, increase the amounts accordingly.
What kind of soil needs amending? Another way to view this is, “have you tried growing without amending? Many soils grow very well with just added nutrients. Typically sands will most benefit from added amendment due to increased water and nutrient holding capacity. I also like to amend clays because they become easier to plant in over time, however the initial go round may be difficult. Clays are very nutritive so amendments may make you feel better gardening in them, but often plants grow very well without amending clay soils. Plant responses to amendments are best in sands.
How often should I amend? This is up to you, but organic matter is “burned up” by the soil microbial community rapidly because 1) you are tilling and this accelerates microbial activity; and 2) you are likely amending during the growing season when warm soil temperatures favor organic matter breakdown. Most gardeners amend prior to replanting the bed.
References
Ferris, H., and M.M. Matute. 2003. Structural and functional succession in the nematode fauna of a soil food web. Applied Soil Ecology 23:93-110
Harris, R.W. Arboriculture: Integrated management of landscape trees, shrubs and vines. 1992. Ed. 2 Prentice-Hall International, New Jersey. 674pp.
Hodel, D.R., Downer, A.J., Pittenger, D.R. and P.J. Beaudoin. 2006. The effect of amended backfill soils when planting five species of palms. HortTechnology 16:457-460.