…it’s an Integrated Pest Management tool!
[Note added after-the-fact: this was a tongue-in-cheek bit of hyperbole – kind of like “it’s not just a Job, it’s an Adventure.” Did not mean to imply that it actually IS an IPM tool. Very badly worded. Hence the beating I took in the comments. Live and learn.]
Fungus gnats (Bradysia spp.) are a pain in the bottom for commercial greenhouse growers. The adults are more of a nuisance than anything else –it just looks bad when a customer picks up your 6” pot of pansies and a bunch of little black gnats take flight. It’s the larvae that are problematic. Adult females lay the eggs in especially damp growing media, and the newly-hatched larvae feed on the roots. There’s both direct damage and also speculation of easier infection of root-borne pathogens, of which there are plenty.

Fungus gnat larvae, just making a living…
Standard control measures include insecticide drenches, biological controls including a specific strain of Bt (Bacillus thuringiensis – sold as GnatrolTM), nematodes, etc. One of the easiest control measures is the one I teach my students: to not over-water, i.e. “grow dry”. But that can be difficult in a big greenhouse range with many different-sized containers, all which drain/dry out at different rates. Propagation houses also have high humidity levels and have to stay moist for rooting/germination purposes and are thus favored by fungus gnats.
Entomologist Dr. Raymond Cloyd of Kansas State University and his group were intrigued by Master Gardener anecdotes of dryer sheets repelling mosquitoes, though no research had been done. Could your common Bounce sheet also repel other pests? And, to take it a step further, what, exactly, repels them? The answers are “yes” and “lots of volatile compounds.”

Their study was published last month in the journal HortScience. Honestly, I’ve never seen descriptors like “controls static cling” and “gives clothes a fresh scent” in a Horticulture journal. Hee! Plus the researchers made it clear this experiment specifically used Bounce Original Outdoor FreshTM. Still kind of humorous, but really good science and the part that’s usually overlooked in the translation to a News Story. Do NOT extrapolate results to include Bounce Spring Fresh, Fresh Linen, and certainly not Downy or Snuggle brands.
The study had a simple design, releasing lab gnats (ha!) into a many-chambered container and observing to which chamber the gnats gravitated to (or away from). There were five different variations on this theme, including an alluringly soggy media sample; when the sample of fabric softener sheet was introduced, they stayed away in droves. All five experiments showed a fairly drastic aversion to the sheet. To determine what was fending off the gnats, they did a steam extraction on sheet samples and ran the condensate through a gas chromatograph – mass spectrometer to measure the volatiles.
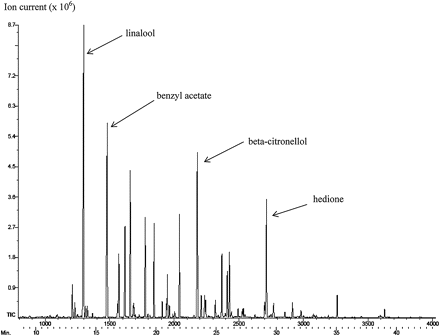
Figure from Bounce® Fabric Softener Dryer Sheets Repel Fungus Gnat, Bradysia sp. nr. coprophila (Diptera: Sciaridae), Adults. Raymond A. Cloyd, Karen A. Marley, Richard A. Larson, and Bari Arieli, HortScience Dec 1 2010: 1830–1833
Well, there you have it. Linalool is a monoterpene alcohol found in lavender, basil, and coriander, and is known to be toxic to mites and insects. Citronello is another monoterpene and lends lemony-freshness to lemon balm, pennyroyal, and rose geranium and has short-term “repellent activity against mosquitoes.” Benzyl acetate, though not specifically mentioned in the results, is another natural fragrance compound, found in jasmine – and is also an industrial-strength solvent. One man’s solvent is another man’s perfume. Or fabric softener. I bet their lab smelled GREAT, by the way.
<
6




 The whole story
The whole story






