Bert’s post yesterday reminded me of some work one of my graduate students did about 10 years ago. We were curious to see whether a transplant fertilizer containing phosphate was correlated with foliar iron deficiency, which is visualized as interveinal chlorosis:

What Scott did was to plant 10 rhododendrons per treatment into pots containing containing a name brand azalea, camellia and rhododendron food (5-5-3) at 0, 0.5, 1.0, and 2.0 times the recommended amount. Here are some of the results of that study:
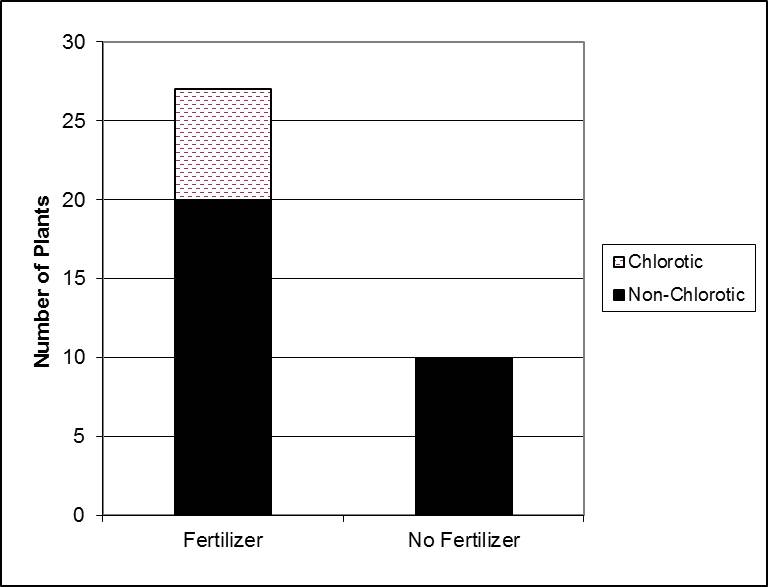
Total number of chlorotic plants
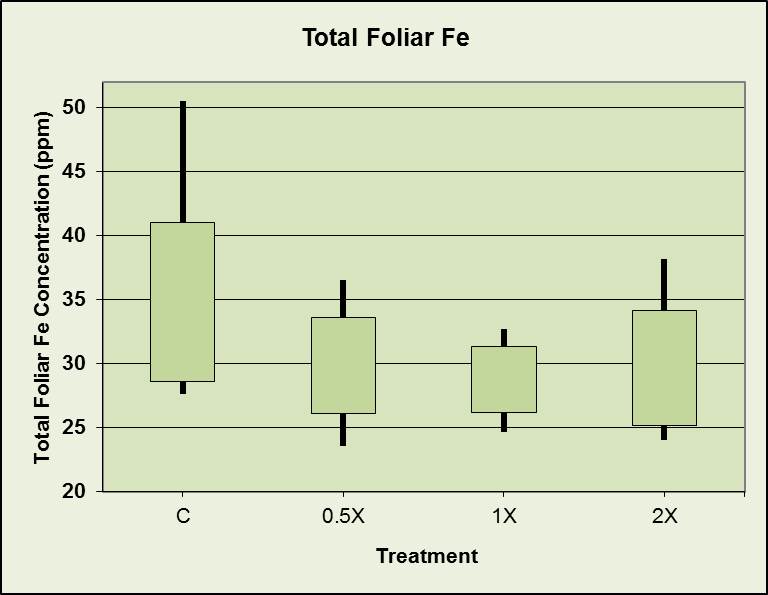 Total foliar iron vs. fertilizer treatment
Total foliar iron vs. fertilizer treatment
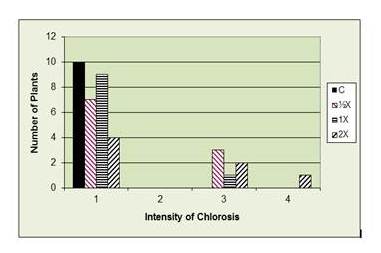 Chlorosis as a result of phosphate fertilizer. 1= Normal (green leaves), 2= Light chlorosis in young leaves, 3= Moderate chlorosis, 4= Severe chlorosis, young leaves white
Chlorosis as a result of phosphate fertilizer. 1= Normal (green leaves), 2= Light chlorosis in young leaves, 3= Moderate chlorosis, 4= Severe chlorosis, young leaves white
For gardeners, the take home message might be that the control plants – those without any transplant fertilizer added – did the best. Don’t add phosphate to your landscape and garden soils unless you have a verified deficiency. And only a soil test will tell you this conclusively.
You can’t fly by the seat of your pants on this one, folks.
I agree with the genral comment that the fertilizer is not required and seems to cause some chlorosis. However, I don’t see the data that puts the blame on excess phosphate?
Robert, this was just a smidgen of the data we generated. The only differences among treatments were the levels of fertilizer used. Neither nitrogen nor potassium interferes with iron uptake – but phosphate does. (I think I’ve blogged about this interference before, but I probably should have repeated that bit.)
Any ideas of what to do when you’ve bought land where all of the plants have this issue because the previous owner added way too much fertilizer?
What happened to the three missing 2X plants? There appears to be only seven in the report.
Terri, they died before the end of the experiment. So we didn’t count them (though that would have made the results even more spectacular). Jen, sorry I didn’t see your older message – but you can plant a cover crop to draw down the nutrients. Remove the cover crop and compost or discard, but don’t till it back in. You will have to add back nutrients like nitrogen.
I may have missed this – but any idea why is the phosphorous excess causing a shut-down of transporters?
Jacquie, it appears to compete with iron for binding sites at the cellular level. The more phosphorus, the less chance iron has to be bound and taken up by the roots.
It’s actually a salt metathesis (double-displacement) reaction that happens in the soil, causing the iron to become an insoluble precipitate. When iron ions come into contact with phosphate ions, they form iron phosphate, which has practically no solubility. It’s just like the classic high school chemistry experiment where a solution of sodium carbonate is added to a solution of copper sulfate, and a precipitate of copper carbonate forms.
(I am currently studying chemistry as a college student, and my biggest hobbies are gardening and plant propagation)
That can make it insoluble in the soil for sure. But P also competes with soluble Fe uptake at the membrane level as well. It’s a double whammy.
Makes sense, since plant roots take up nutrients primarily by ion exchange*, but nothing says that at any given time, the plant can only take up anions or cations. They can take up both, so long as they are replaced with H+ and OH- accordingly. Since the iron doesn’t normally immobilize until it is converted to chlorophyll, which is the whole reason the plant needs it, in order for that, the iron needs to remain soluble up until then. When there’s too much phosphate, however, the iron immobilizes prematurely, so the chlorophyll isn’t getting made, and the plant goes chlorotic.
*When they take up nutrient cations, they do so by replacing each unit of positive charge with a H+ ion, which is obviously another cation, but when they take up anions (like phosphate), they replace each unit of negative charge with a OH- ion, which is obviously another anion.
For cations:
For each NH4+ ion the plant takes from the soil, one H+ is returned in its place. For each Fe+2, Mg+2, Ca+2, Zn+2, Mn+2, Cu+2, and Ni+2 ion the plant takes from the soil, two H+ ions are added to the soil to equalize charge.
For anions:
For each NO3- ion taken up by a plant, one OH- is added to the soil to equalize charge.
For each PO4 -3 ion taken up, three OH- ions are used to equalize charge.
You’re looking at this from strictly a soil chemistry perspective. What you need to add is the influence of both roots and mycorrhizae, which acidify the rhizosphere and enable metal solubilization. The problem for plants is the competition for membrane uptake sites – not soil immobilization.
good is very good
Hi, I’m coming to these comments a few years later. We moved to this house a couple of years ago and everything I have planted in a previously fenced area, that is sensitive to iron deficiency, has been chlorotic, be they trees, shrubs, perennials and annuals. And the soil phosphorus test was literally almost off-the-charts high. The area, before we bought it, for the last at least 15 years, has been a partly shaded, grassy dog run area, maybe 30X30 ft. All I can think of is that having dogs there for so long could have boosted the phosphorus levels but I can’t find anything on that subject. Based on what happens when excess livestock manure is applied to crop land, would this hypothesis make sense?
Do you have any ideas how many years I would be looking at needing to foliar feed iron to this area? Before recognizing the extent of the problem, I have created a lovely enclosed ornamental garden space and taking everything out to plant cover crops to take up the phosphorus isn’t really an option at this point.
I’m grateful to have finally found someone who can help me figure this out! Thank you.
1. Did you have a lab soil test done or was it a DIY test?
2. Don’t try to boost soil Fe levels with fertilizer or foliar applications.
3. To reduce excess soil nutrients keep planting cover crops and then harvest them, just like you’ve been doing. Plants will reduce excess soil nutrients quite well and without causing other nutrient issues.
4. It’s doubtful the dogs had much if anything to do with the excessive P levels you’re finding.It’s usually caused by too much fertilizer or the overuse of compost. Use neither until a soil test shows the P levels are acceptable.
5. Does your area or region have an issue with excessive phosphorus in the soil in the first place?
Also, as I want to keep top dressing with organic matter over time, can I use composted goat manure/bedding mixture. Or, in other words, does phosphorus remain in any composted manure and I should stay away from that? Thank you.
Unless you have a soil test that shows you have (1) a deficiency in major nutrients and (2) a low percent organic matter, you should not use compost. It’s “fast food” and yes, the phosphorus is there and readily available. Arborist wood chips are a much better choice as they are a low and slow source of nutrients.
Thank you, Linda.